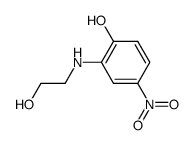
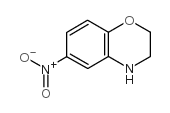
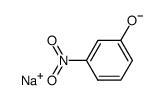
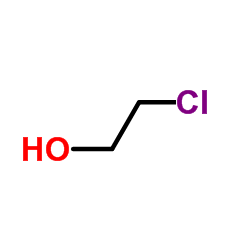
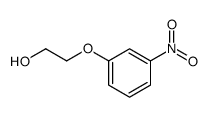
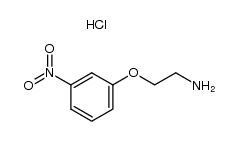
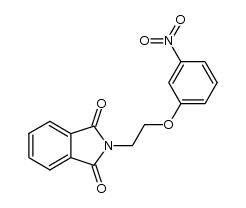
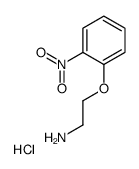
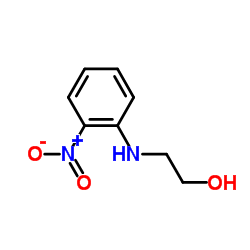
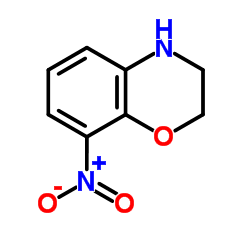
|
~13% |
|
~45% |
|
~73% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~58% |
|
~% |
|
~18% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~10% |
|
~71% |
|
~87% |
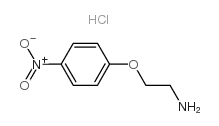
![2-[(4-Nitrophenyl)amino]ethanol Structure](https://image.chemsrc.com/caspic/328/1965-54-4.png)