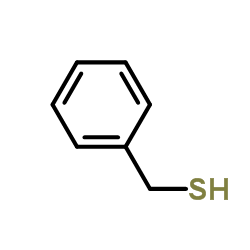
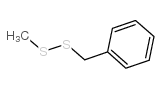
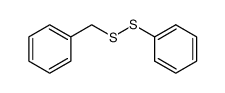
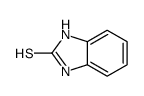
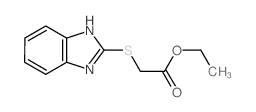
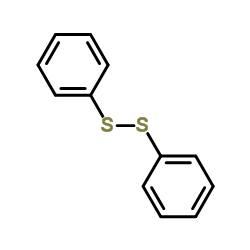
|
~78% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~0% |
|
~0% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~67% |
|
~0% |
|
~0% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
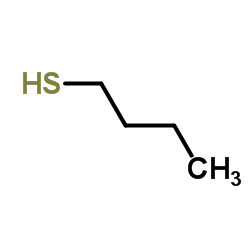

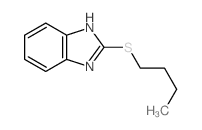
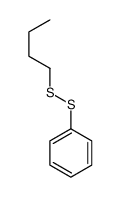

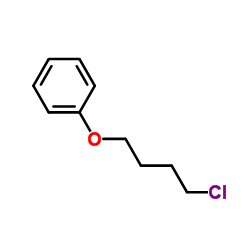
![2-((4-phenoxybutyl)thio)-1H-benzo[d]imidazole Structure](https://image.chemsrc.com/caspic/219/110143-83-4.png)
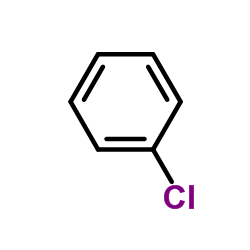

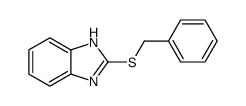
![1H-Benzimidazole, 2-[(R)-methylsulfinyl] Structure](https://image.chemsrc.com/caspic/216/98639-91-9.png)
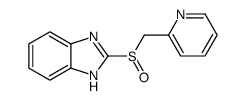
![2-{[(pyridin-2-yl)methyl]thio}-1H-benzimidazole Structure](https://image.chemsrc.com/caspic/446/23593-22-8.png)
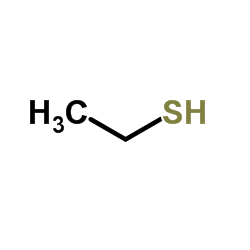
![2-(Ethylthio)-1H-Benzo[d]Imidazole Structure](https://image.chemsrc.com/caspic/262/14610-11-8.png)