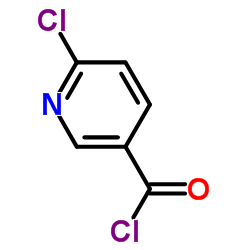
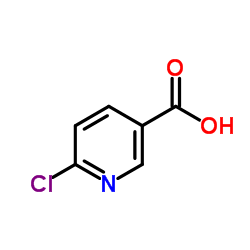
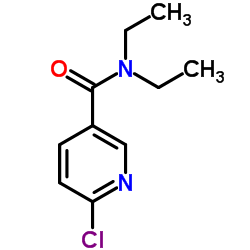
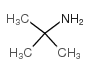
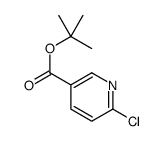
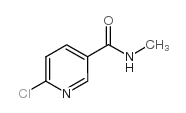
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~96% |
|
~39% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~97% |
|
~% |
|
~% |
|
~% |
|
~87% |
|
~% |
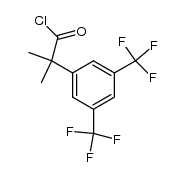
![N-methyl-N-[6-(morpholin-4-yl)-4-(o-tolyl)pyridin-3-yl]amine Structure](https://image.chemsrc.com/caspic/300/290297-36-8.png)
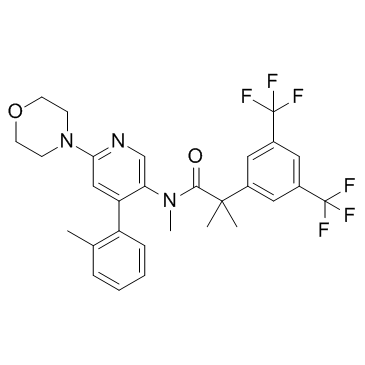

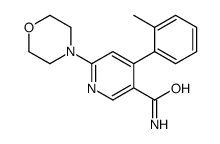

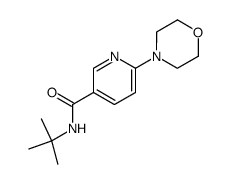
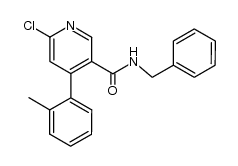
![[6-(morpholin-4-yl)-4-(o-tolyl)pyridin-3-yl]carbamic acid methyl ester Structure](https://image.chemsrc.com/caspic/440/342417-07-6.png)

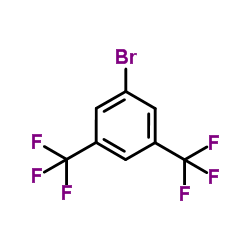
![2-[3,5-bis-(Trifluoromethyl)phenyl]propan-2-ol Structure](https://image.chemsrc.com/caspic/401/67570-38-1.png)
![2-[3,5-bis(trifluoromethyl)phenyl]-2-methylpropanoic acid Structure](https://image.chemsrc.com/caspic/424/289686-70-0.png)