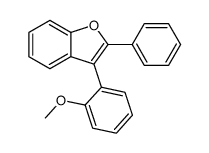
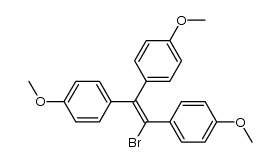
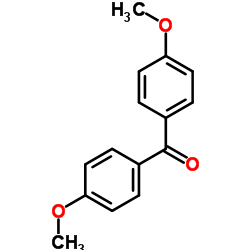
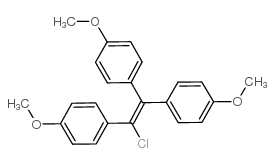
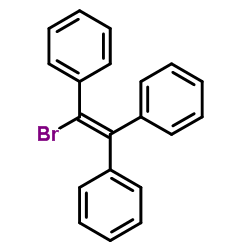
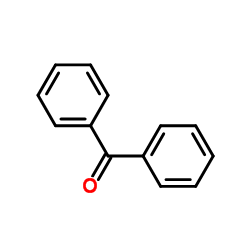
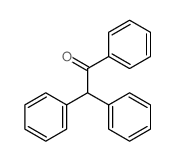
|
~60% |
|
~18% |
|
~0% |
|
~3% |
|
~0%
Detail
|
|
~20% |
|
~11% |
![1-[2-bromo-1-(2-methoxyphenyl)-2-phenylethenyl]-2-methoxybenzene Structure](https://image.chemsrc.com/caspic/200/62378-32-9.png)
![1-methoxy-2-[1-(2-methoxyphenyl)-2-phenylethenyl]benzene Structure](https://image.chemsrc.com/caspic/424/62378-39-6.png)