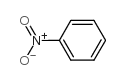
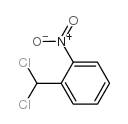
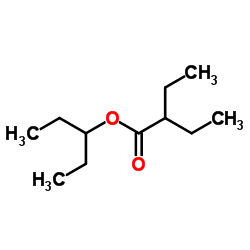
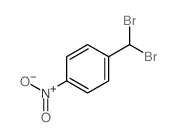
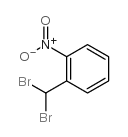
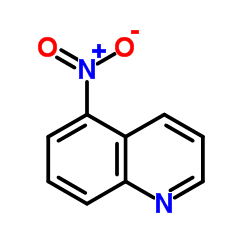
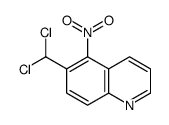
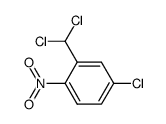
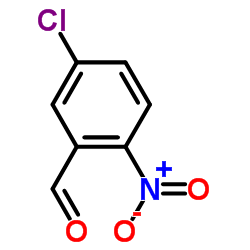
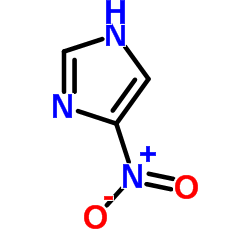
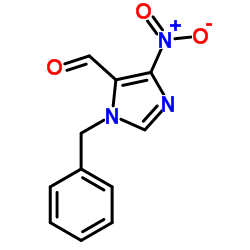
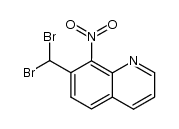
|
~13% |
|
~16% |
|
~15% |
|
~92% |
|
~60% |
|
~53% |
|
~89% |
|
~52% |
|
~89% |
|
~98% |