|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

![[dimethyl(phenyl)silyl]-ethenyl-dimethylsilane Structure](https://image.chemsrc.com/caspic/460/61499-43-2.png)
![2-[dimethyl(phenyl)silyl]ethyl-methoxy-dimethylsilane Structure](https://image.chemsrc.com/caspic/422/61499-44-3.png)

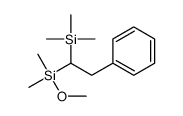
![ethenyl-[2-[methoxy(dimethyl)silyl]ethyl]-dimethylsilane Structure](https://image.chemsrc.com/caspic/285/61245-01-0.png)

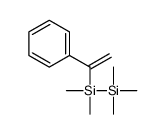
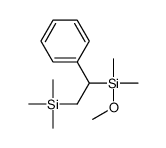
![dimethyl-[(E)-2-phenylethenyl]-trimethylsilylsilane Structure](https://image.chemsrc.com/caspic/464/40595-36-6.png)