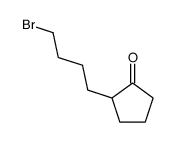
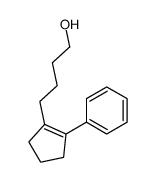
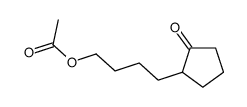
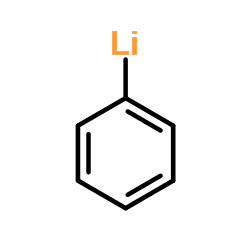
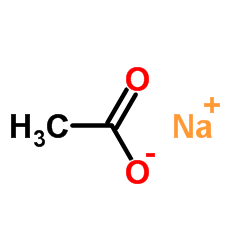
|
~% |
|
~% |
|
~% |
|
~% |
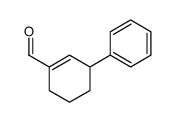
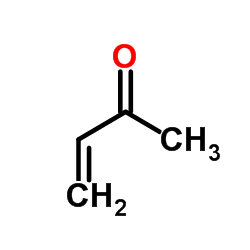
![10-phenylspiro[5.5]undeca-4,10-dien-3-one Structure](https://image.chemsrc.com/caspic/324/61114-29-2.png)