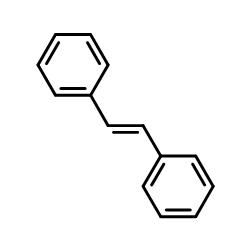
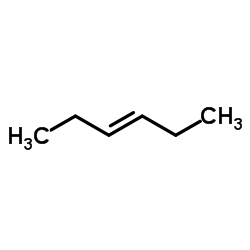
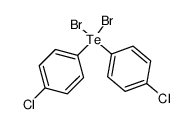
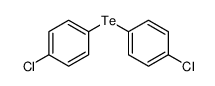
|
~% |
|
~% |
|
~% |
|
~% |
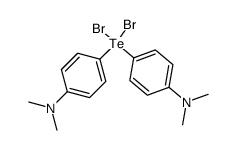
![4-[4-(dimethylamino)phenyl]tellanyl-N,N-dimethylaniline Structure](https://image.chemsrc.com/caspic/423/59130-74-4.png)