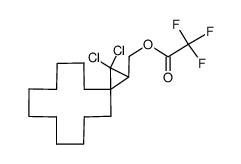
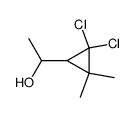
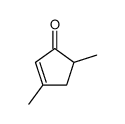
|
~57% |
|
~89% |
|
~70% |
|
~87% |
|
~74% |
|
~% |
|
~90% |
|
~75% |
|
~85% |
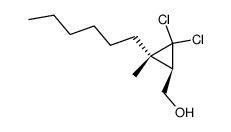
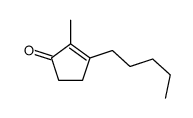


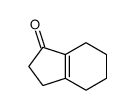





![2,3,4,5,6,7,8,9,10,11,12,13-Dodecahydro-1H-cyclopenta[12]annulen- 1-one Structure](https://image.chemsrc.com/caspic/178/15210-25-0.png)
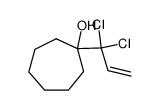
![(2,2-dichlorospiro[2.11]tetradecan-1-yl)methanol Structure](https://image.chemsrc.com/caspic/226/52815-18-6.png)