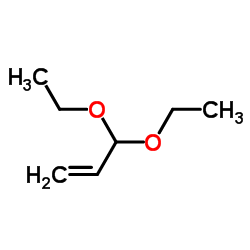
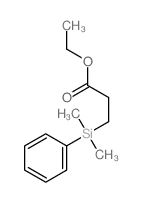
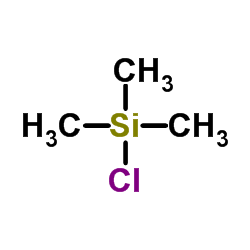
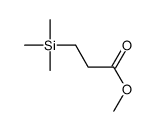
|
~% |
|
~% |
|
~% |
|
~% |
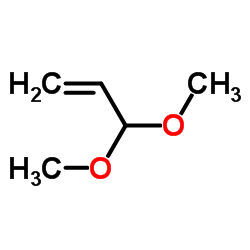
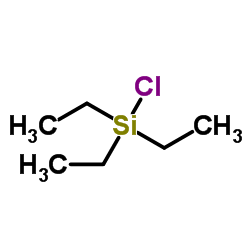
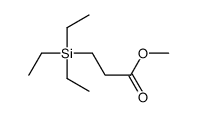

![methyl 3-[dimethyl(phenyl)silyl]propanoate Structure](https://image.chemsrc.com/caspic/047/59344-04-6.png)