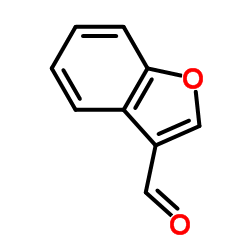
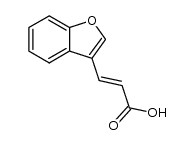
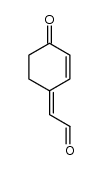
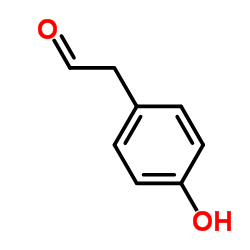
|
~79% |
|
~99% |
|
~% |
|
~% |
|
~% |
|
~79% |
|
~% |
|
~% |
|
~% |
|
~87% |
|
~% |

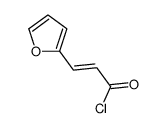
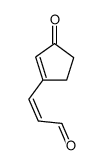
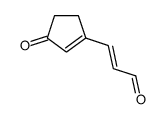
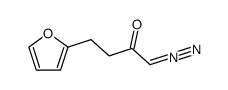
![1,2,3a,3b-tetrahydro-3H-benzo[b]cyclopenta[1,3]cyclopropa[1,2-d]furan-3-one Structure](https://image.chemsrc.com/caspic/269/105456-85-7.png)
![[1,1-Biphenyl]-2,4-diol Structure](https://image.chemsrc.com/caspic/039/611-62-1.png)