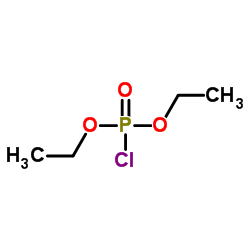
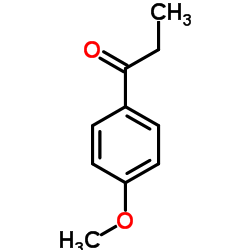
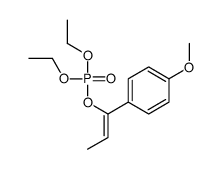
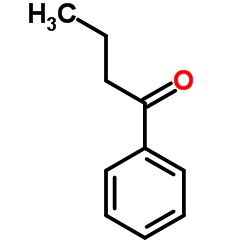
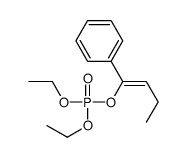
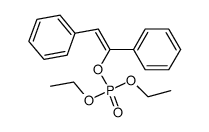
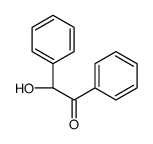
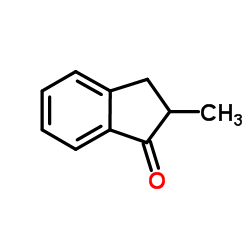
|
~85% |
|
~90% |
|
~14% |
|
~85% |