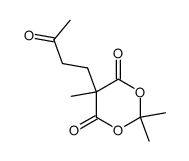
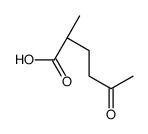
|
~70% |
|
~81% |
|
~% |
|
~82% |
|
~74% |
![2-Naphthalenol,1-[(dimethylamino)methyl] Structure](https://image.chemsrc.com/caspic/102/5419-02-3.png)

![2-phenyl-1,2-dihydrobenzo[f]chromen-3-one Structure](https://image.chemsrc.com/caspic/062/52600-63-2.png)
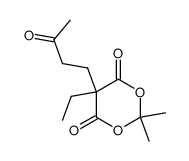
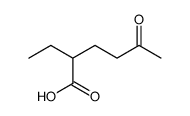


![isopropylidene benzyl[(3-indolyl)methyl]malonate Structure](https://image.chemsrc.com/caspic/049/82431-05-8.png)