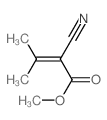
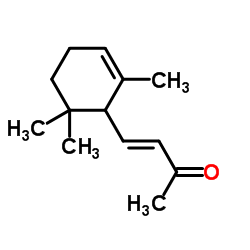
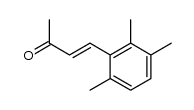
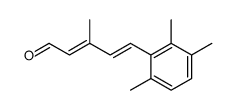
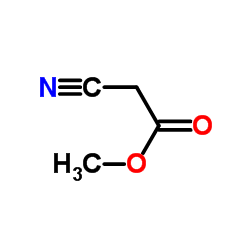
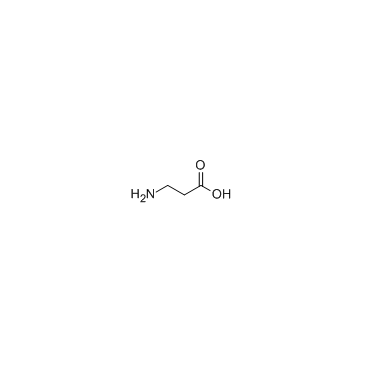
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~47% |

![1,2,4-trimethyl-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,3,6-trimethylphenyl)octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]benzene Structure](https://image.chemsrc.com/caspic/136/524-01-6.png)