|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

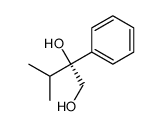
![(1S,2R,3S,5R,2'S)-6,6-dimethyl-3-(2'-ethoxy-1'-phenyl-1'-oxo-2'-ethylsulfanyl)-2-hydroxymethyl-bicyclo[3.1.1]heptane Structure](https://image.chemsrc.com/caspic/233/682352-08-5.png)
![(1S,2R,3S,5R,2'S)-6,6-dimethyl-3-(2'-ethoxy-1'-phenyl-1'-oxo-2'-ethylsulfanyl)-2-(O-t-butyl-dimethylsilyl-hydroxymethyl)-bicyclo[3.1.1]heptane Structure](https://image.chemsrc.com/caspic/340/682352-10-9.png)
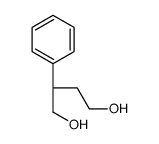

![(1S,2R,3S,5R,2'R)-6,6-dimethyl-3-(2'-ethoxy-1'-phenyl-1'-oxo-2'-ethylsulfanyl)-2-(O-t-butyl-dimethylsilyl-hydroxymethyl)-bicyclo[3.1.1]heptane Structure](https://image.chemsrc.com/caspic/292/682352-11-0.png)