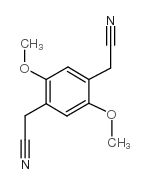
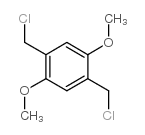
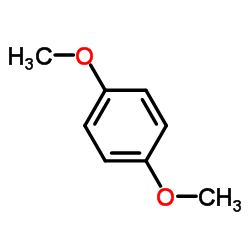
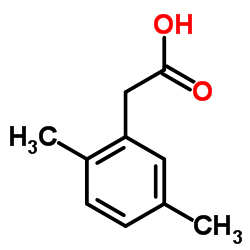
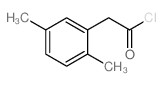
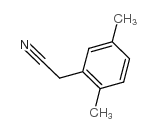
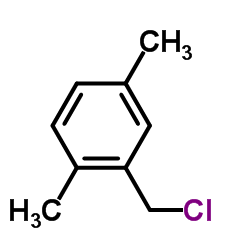
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![2-[4-(carboxymethyl)-2,5-dimethoxy-phenyl]acetic acid Structure](https://image.chemsrc.com/caspic/332/38439-92-8.png)
![2-[4-(2-chloro-2-oxoethyl)-2,5-dimethoxyphenyl]acetyl chloride Structure](https://image.chemsrc.com/caspic/041/51053-93-1.png)