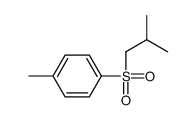
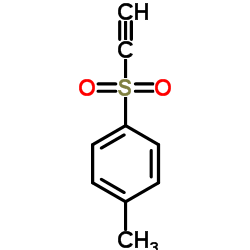
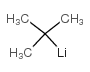
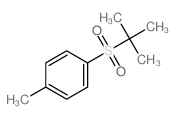
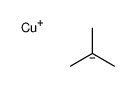
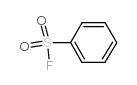
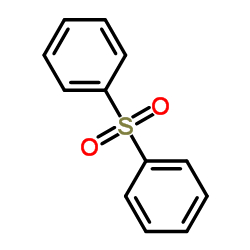
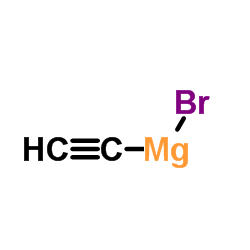|
~31% |
|
~92% |
|
~82% |
|
~77% |
|
~85% |
|
~99% |
|
~20% |
|
~94% |
|
~10% |
|
~89% |
|
~12% |
|
~60% |
|
~92% |
|
~87% |
|
~73% |
|
~13% |
|
~12% |
|
~17% |
|
~95% |
|
~69% |
|
~17% |
|
~69% |
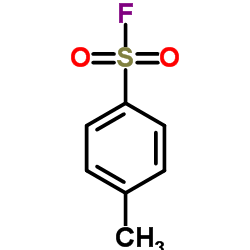
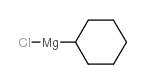
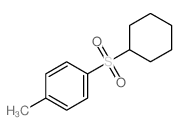
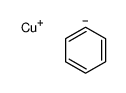
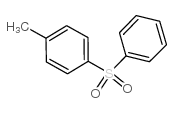
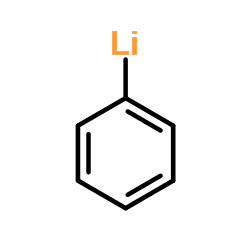
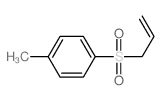
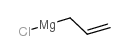
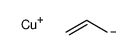

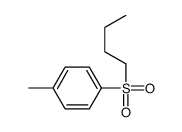


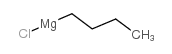


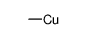
![1-methyl-4-[2-methyl-1-(4-methylphenyl)sulfonylpropyl]sulfonylbenzene Structure](https://image.chemsrc.com/caspic/440/72834-71-0.png)