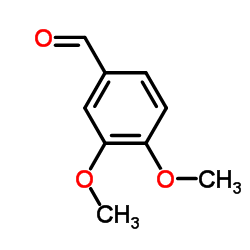
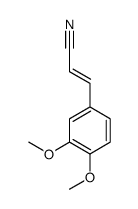
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
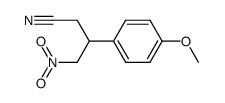
![4-[2-(dimethylamino)ethyl]-4-(4-methoxyphenyl)cyclohex-2-en-1-one Structure](https://image.chemsrc.com/caspic/147/34603-52-6.png)
![2-(9-(4-methoxyphenyl)-1,5-dithiaspiro[5.5]undec-7-en-9-yl)-N,N-dimethylethan-1-amine Structure](https://image.chemsrc.com/caspic/258/79215-00-2.png)
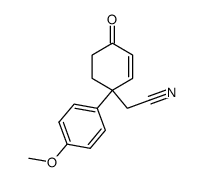
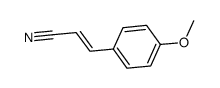


![2-(9-(4-methoxyphenyl)-1,5-dithiaspiro[5.5]undec-7-en-9-yl)acetaldehyde Structure](https://image.chemsrc.com/caspic/417/79214-98-5.png)
![2-(9-(4-methoxyphenyl)-1,5-dithiaspiro[5.5]undec-7-en-9-yl)acetonitrile Structure](https://image.chemsrc.com/caspic/493/79214-96-3.png)