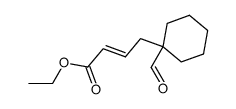
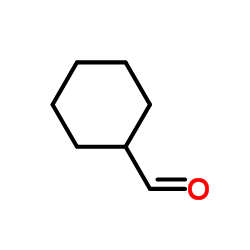
|
~43% |
|
~% |
|
~% |
|
~% |
|
~% |

![SPIRO[4.4]NONAN-2-ONE Structure](https://image.chemsrc.com/caspic/244/34177-18-9.png)