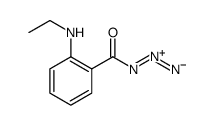
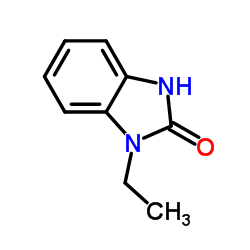
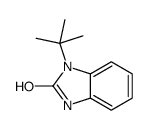
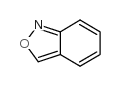
|
~85% |
|
~81% |
|
~77% |
|
~95% |
|
~% |
|
~74% |
|
~53% |
|
~79% |
![7-tert-butyl-7-azabicyclo[4.2.0]octa-1,3,5-trien-8-one Structure](https://image.chemsrc.com/caspic/397/31562-07-9.png)
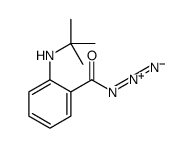
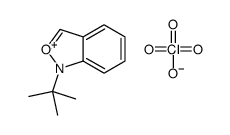
![[2-(tert-butylamino)benzoyl] 2-(tert-butylamino)benzoate Structure](https://image.chemsrc.com/caspic/186/61752-04-3.png)

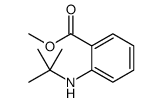
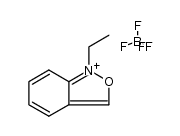
![7-Azabicyclo[4.2.0]octa-1,3,5-trien-8-one,7-ethyl-(9CI) Structure](https://image.chemsrc.com/caspic/244/91201-94-4.png)