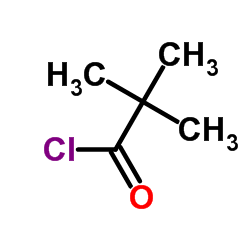
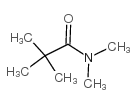
|
~36% |
|
~% |
|
~93% |
|
~41% |
|
~% |
|
~% |
|
~45% |
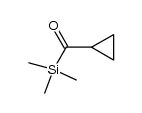
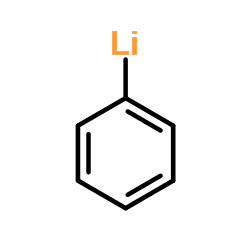
![[cyclopropyl(phenyl)methoxy]-trimethylsilane Structure](https://image.chemsrc.com/caspic/163/98603-79-3.png)
![(2-cyclopropyl[1,3]dithian-2-yl)trimethylsilane Structure](https://image.chemsrc.com/caspic/096/851225-51-9.png)
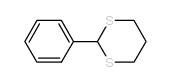

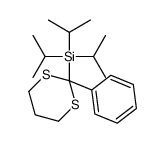
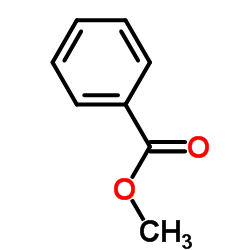
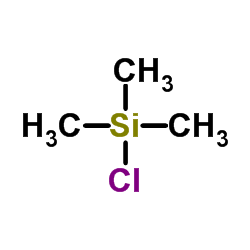
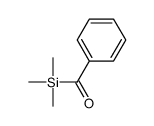

![[dimethyl(phenyl)silyl]-phenylmethanone Structure](https://image.chemsrc.com/caspic/214/17909-51-2.png)