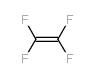
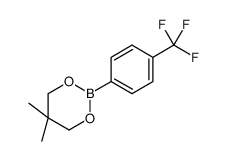
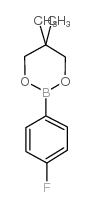
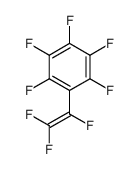
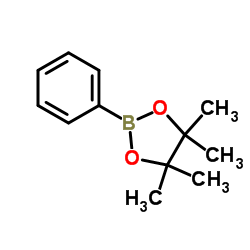
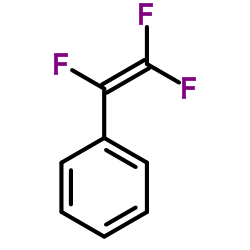
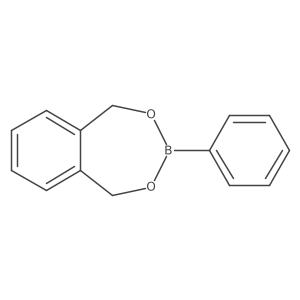
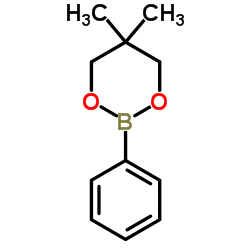
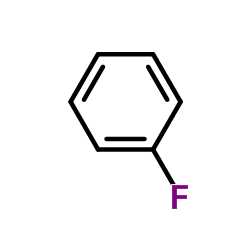
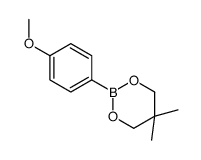
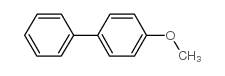
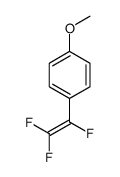
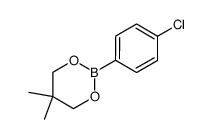
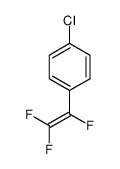
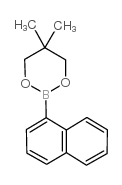
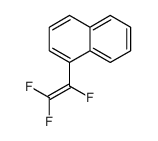
|
~30% |
|
~22% |
|
~50% |
|
~23% |
|
~64% |
|
~58% |
|
~86% |
|
~19% |
|
~55% |
|
~43% |
|
~63% |