|
~31% |
|
~85% |
|
~% |
|
~95% |
|
~% |
|
~92% |
|
~% |
|
~% |
|
~66% |
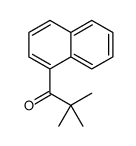

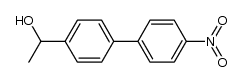
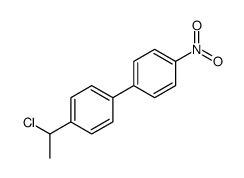
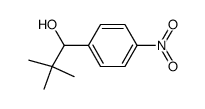
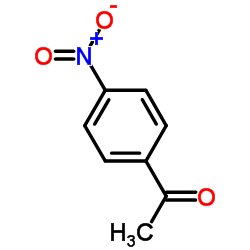
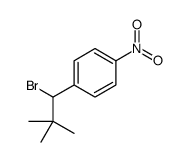
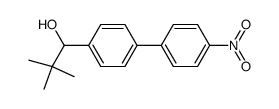

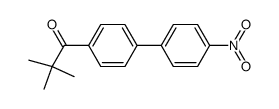
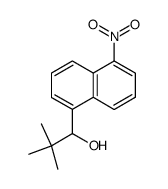
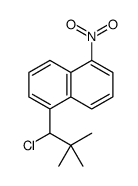

![1-[4-(bromomethyl)phenyl]-2,2-dimethylpropan-1-one Structure](https://image.chemsrc.com/caspic/147/52449-32-8.png)