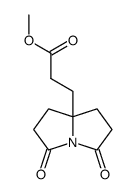
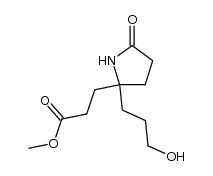
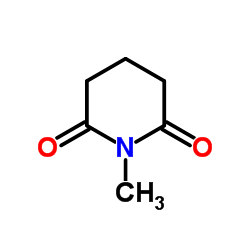
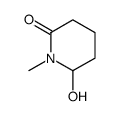
|
~72% |
|
~7% |
|
~7% |
|
~51% |
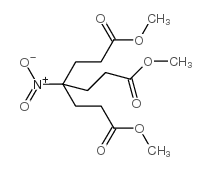
![methyl 3-[2-(3-methoxy-3-oxopropyl)-5-oxopyrrolidin-2-yl]propanoate Structure](https://image.chemsrc.com/caspic/433/89317-31-7.png)