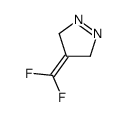
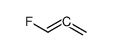
|
~74% |
|
~50% |
|
~32% |
|
~% |
|
~% |
|
~19% |
|
~53% |
|
~64% |


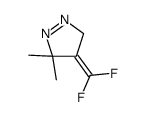
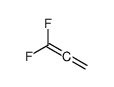
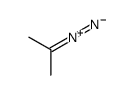
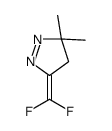


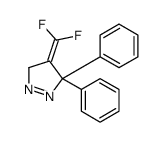
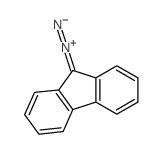
![2-(difluoromethylidene)spiro[cyclopropane-1,9'-fluorene] Structure](https://image.chemsrc.com/caspic/210/89210-61-7.png)