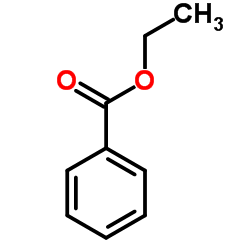
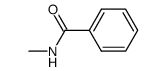
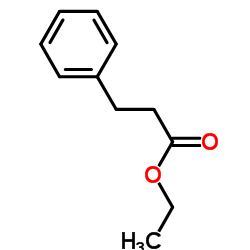
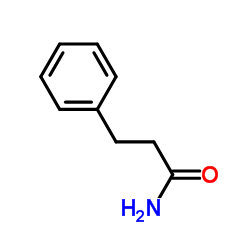
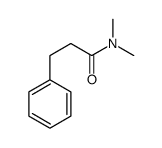
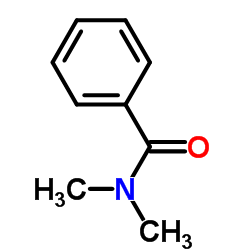
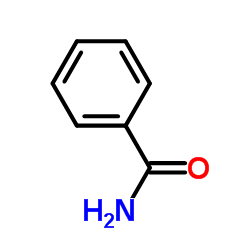
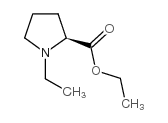
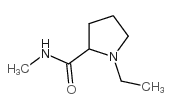
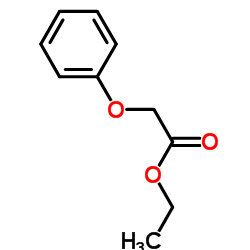
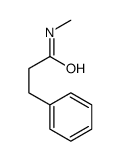
|
~77% |
|
~88% |
|
~75% |
|
~39% |
|
~65% |
|
~84% |
|
~48% |
|
~83% |