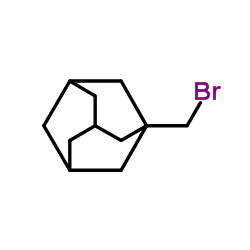
|
~51%
Detail
|
|
~77% |
|
~% |
|
~1% |
|
~% |
|
~% |
|
~% |
|
~29% |
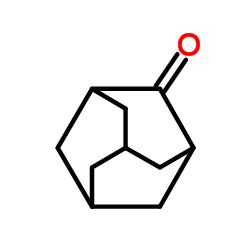
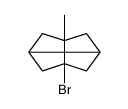
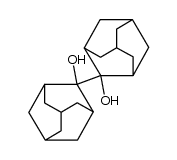

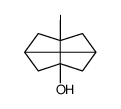
![7-Methylenebicyclo[3.3.1]nonan-3-one Structure](https://image.chemsrc.com/caspic/376/17933-29-8.png)

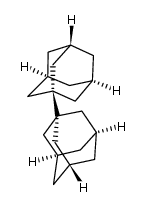
![Tricyclo[4.3.1.13,8]undecan-3-ol Structure](https://image.chemsrc.com/caspic/422/14504-80-4.png)
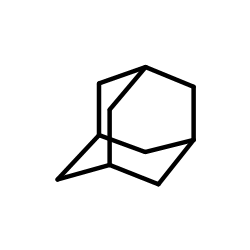
![3-Bromotricyclo[4.3.1.13,8]undecane Structure](https://image.chemsrc.com/caspic/270/14504-84-8.png)