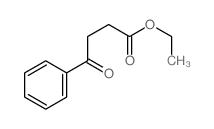
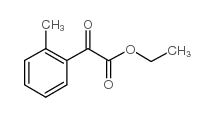
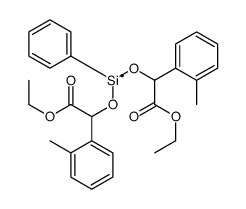
|
~37% |
|
~47% |
|
~53% |
|
~54% |