Novel tetra 4-(hydroxymethyl)-2,6-dimethoxyphenoxyl substituted metallophthalocyanines: Synthesis, electrochemical redox, electrocatalytic oxygen reducing, and volatile organic compounds sensing and adsorption properties
İpek Günay, Efe Baturhan Orman, Ahmet Altındal, Bekir Salih, Metin Özer, Ali Rıza Özkaya
Index: 10.1016/j.dyepig.2018.02.037
Full Text: HTML
Abstract
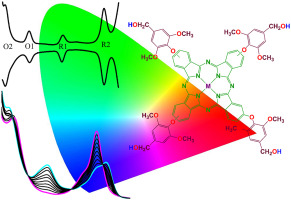
Novel tetrakis [(4-(hydroxymethyl)-2,6-dimethoxyphenoxyl)] substituted zinc(II), cobalt(II) and iron(II) phthalocyanines were synthesized by the reaction of (4-(hydroxymethyl)-2,6-dimethoxyphenoxy)phthalonitrile with suitable metal salts in 2-N, N-dimethylaminoethanol. The phthalonitrile ligand was prepared from 4-hydroxy-3,5-dimethoxy benzyl alcohol and 4-nitrophthalonitrile using potassium carbonate as catalyst in N,N-dimethylformamide at 50 °C. The structural characterization of the compounds was carried out by elemental analysis, FTIR, UV–vis and MALDI-TOF Mass spectroscopic methods. Electron transfer properties and electrocatalytic oxygen reducing performances of the compounds were also investigated by electrochemical, in situ spectroelectrochemical and in situ electrocolorimetric measurements. Electrochemical, in situ spectroelectrochemical, and in situ electrocolorimetric measurements showed that rich redox behavior of the complexes leads to their high electrocatalytic activity for oxygen reduction and net electrocolorimetric changes suitable for electrochromic applications. Phthalocyanine central metal ion effect on the sensing properties and the adsorption kinetics of four main groups of volatile organic compounds (alkanes, alcohols, chlorinated hydrocarbons and amines) onto these novel compounds were also examined by three different models. While the sensitivity of the sensors was strongly dependent on the nature of the metal ion, no considerable effect was observed on the adsorption kinetics. The evaluation of the kinetics of adsorption processes with respect to three different models showed that pseudo second order rate equation best describes the adsorption of alcohols and chlorinated hydrocarbons while the adsorption of alkanes and amines on these compounds can be represented by Elovich equation.
|
Thermal stable blue pigment with tunable color of DyIn1-xMnx...
2018-04-10 [10.1016/j.dyepig.2018.03.051] |
|
Nitrogen introduction of spirobifluorene to form α-, β-, γ-,...
2018-04-03 [10.1016/j.dyepig.2018.03.066] |
|
Solution processable fluorene-extended Indeno[1,2-b]anthrace...
2018-04-03 [10.1016/j.dyepig.2018.03.076] |
|
(D−π−A)3−Type metal-free organic dye for dye-sensitized sola...
2018-04-01 [10.1016/j.dyepig.2018.03.075] |
|
Effect of morphology on the near infrared shielding property...
2018-03-31 [10.1016/j.dyepig.2018.03.074] |