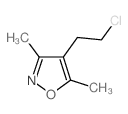
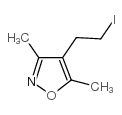
|
~78% |
|
~% |
|
~10% |
|
~% |
|
~32% |
|
~0% |
|
~63% |
|
~58% |
|
~53% |
|
~95% |
|
~77% |
|
~% |
|
~56% |
|
~82% |
|
~53% |
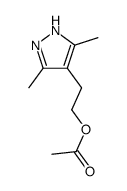
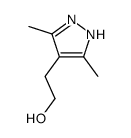
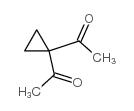
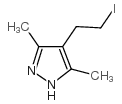
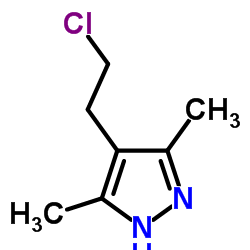
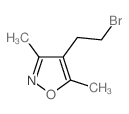
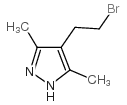
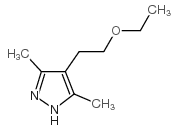


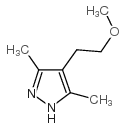
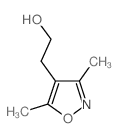

![4,7-dimethyl-5-oxa-6-azaspiro[2.4]hept-6-en-4-ol Structure](https://image.chemsrc.com/caspic/359/83467-46-3.png)