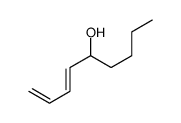
|
~92% |
|
~86% |
|
~75% |
|
~83% |
|
~77% |
|
~71% |
|
~70% |
![1-((1R,4R,7S)-2,2-dioxido-1,3,3a,4,7,7a-hexahydro-4,7-methanobenzo[c]thiophen-1-yl)pentan-1-one Structure](https://image.chemsrc.com/caspic/404/93469-35-3.png)
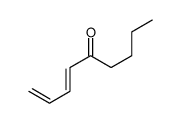
![(1S,3aR,4S,7R,7aS)-1-(trimethylsilyl)-1,3,3a,4,7,7a-hexahydro-4,7-methanobenzo[c]thiophene 2,2-dioxide Structure](https://image.chemsrc.com/caspic/405/86593-81-9.png)

![1-((1S,3R,7R)-4,4-Dioxo-4λ6-thia-tricyclo[5.2.1.02,6]dec-8-en-3-yl)-propan-1-ol Structure](https://image.chemsrc.com/caspic/432/88920-63-2.png)
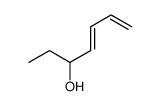
![(1S,4R,7S)-1-methyl-1,3,3a,4,7,7a-hexahydro-4,7-methanobenzo[c]thiophene 2,2-dioxide Structure](https://image.chemsrc.com/caspic/044/83927-50-8.png)


![(1R,4R,7S)-1-((R)-1-hydroxypentyl)-1,3,3a,4,7,7a-hexahydro-4,7-methanobenzo[c]thiophene 2,2-dioxide Structure](https://image.chemsrc.com/caspic/394/88920-64-3.png)