Higher Alcohols Synthesis over Carbon Nanohorn-Supported KCoRhMo Catalyst: Pelletization and Kinetic Modeling
Philip E. Boahene, Ajay K. Dalai
Index: 10.1021/acs.iecr.7b03760
Full Text: HTML
Abstract
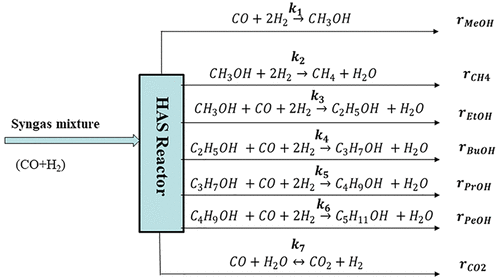
In this study, two catalyst grain sizes (fine powders and pellets) have been investigated to elucidate the effects of both external and internal mass transfer diffusion as well as particle size during the CO hydrogenation reaction to produce higher alcohols. Catalyst grain sizes of 88 and 254 μm were advisedly chosen based on our previous investigations with a similar catalyst matrix for the higher alcohol synthesis (HAS) reaction. The focus in this work was to explore the attractive textural properties of CNH support for CO hydrogenation reaction to produce higher alcohols under specified reaction conditions. Also, to ascertain the possibilities of commercialization of the developed carbon nanohorn (CNH)-supported KCoRhMo catalyst, bentonite clay was added to the pristine support as a binder to enhance its mechanical/crushing strength and kinetic analyses of such catalyst assessed. The influence of bentonite clay was investigated by incorporating 5 wt % of this binder into the formulation of the KCoRhMo/CNH catalyst. For mass transfer considerations, the finely ground KCoRhMo/CNH catalyst powder of 88 μm particle size was used so as to eradicate mass transfer resistance. CO hydrogenation experiments were carried out using a two-phase fixed-bed reactor system to ascertain the intrinsic kinetics of the liquid alcohol products (methanol, ethanol, higher alcohols) as well as the gaseous products generated by the reaction. Syngas of H2/CO ratio of 1.25 was used as feedstock at temperatures, pressures, and gas hourly space velocity ranges of 290–350 °C, 800–1400 psig, and 2.4–4.8 m3 STP/kgcat/h, respectively. The power law model was used to fit experimental data to evaluate kinetic parameters for the HAS reaction on the catalyst surface. Fitting of the experimental data with the developed power law models showed good fits with high R2 values in the range of 0.88–0.96 for the components evaluated. The activation energies computed for ethanol and propanol in the HAS reaction over CNH-supported KCoRhMo catalyst were 54.4 and 92.2 kJ/mol, which are low compared to values obtained by other researchers in similar studies.
|
Reaction Pathways and Microkinetic Modeling of n-Butane Oxid...
2018-04-16 [10.1021/acs.iecr.8b00589] |
|
Vapor Pressure and Heat of Vaporization of Molecules That As...
2018-04-16 [10.1021/acs.iecr.7b04241] |
|
Dual SIMC-PI Controller Design for Cascade Implement of Inpu...
2018-04-13 [10.1021/acs.iecr.7b05047] |
|
Adsorption of Contaminants of Emerging Concern from Aqueous ...
2018-04-13 [10.1021/acs.iecr.7b05168] |
|
Feasibility evaluation of a novel middle vapor recompression...
2018-04-13 [10.1021/acs.iecr.8b00038] |