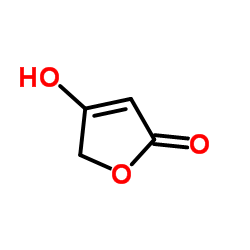
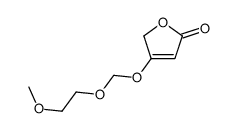
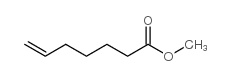
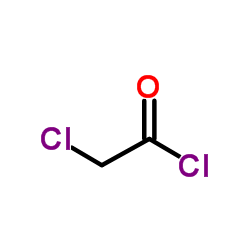
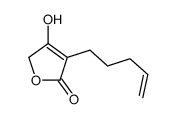
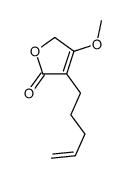
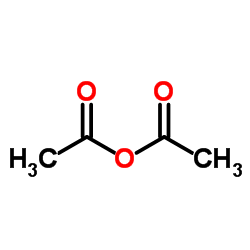
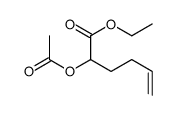
|
~87% |
|
~27% |
|
~91% |
|
~% |
|
~82% |
|
~95% |
|
~% |
|
~% |