|
~65% |
|
~% |
|
~% |
|
~65% |
|
~% |
|
~% |
|
~68% |
|
~% |
|
~% |
|
~72% |
|
~% |
|
~% |
|
~69% |
|
~% |
|
~% |
|
~72% |
|
~% |
|
~% |
|
~82% |
|
~% |
|
~% |
|
~67% |
|
~% |
|
~% |
|
~65% |
|
~% |
|
~% |
|
~72% |
|
~% |
|
~% |

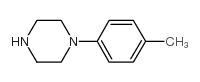

![N-[(E)-[1-[[4-(4-methylphenyl)piperazin-1-yl]methyl]-2-oxoindol-3-ylidene]amino]-2-naphthalen-2-yloxyacetamide Structure](https://image.chemsrc.com/caspic/151/81215-52-3.png)
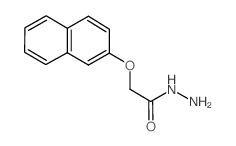
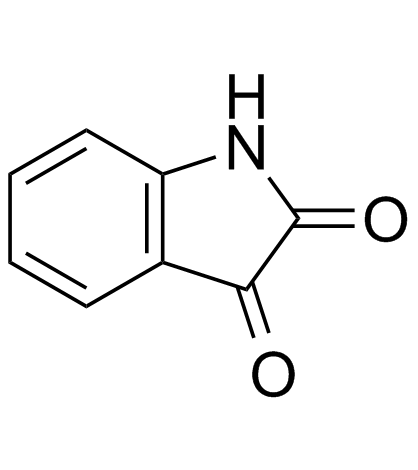
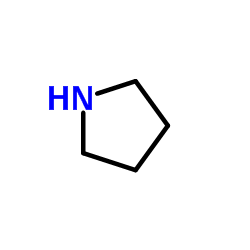
![[2]naphthyloxy-acetic acid-(5-methyl-2-oxo-indolin-3-ylidenehydrazide) Structure](https://image.chemsrc.com/caspic/168/81215-47-6.png)
![N-[[5-methyl-2-oxo-1-(pyrrolidin-1-ylmethyl)indol-3-ylidene]amino]-2-naphthalen-2-yloxyacetamide Structure](https://image.chemsrc.com/caspic/113/81215-53-4.png)

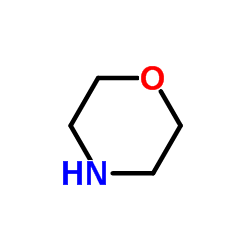
![N-[[5-methyl-1-(morpholin-4-ylmethyl)-2-oxoindol-3-ylidene]amino]-2-naphthalen-2-yloxyacetamide Structure](https://image.chemsrc.com/caspic/075/81215-54-5.png)

![N-[(E)-[5-methyl-2-oxo-1-(piperidin-1-ylmethyl)indol-3-ylidene]amino]-2-naphthalen-2-yloxyacetamide Structure](https://image.chemsrc.com/caspic/037/81215-55-6.png)
![2-naphthalen-2-yloxy-N-[(E)-[2-oxo-1-(piperidin-1-ylmethyl)indol-3-ylidene]amino]acetamide Structure](https://image.chemsrc.com/caspic/160/81315-43-7.png)
![N-[(E)-[5-methyl-1-[[4-(4-methylphenyl)piperazin-1-yl]methyl]-2-oxoindol-3-ylidene]amino]-2-naphthalen-2-yloxyacetamide Structure](https://image.chemsrc.com/caspic/499/81215-56-7.png)

![N-[[1-(morpholin-4-ylmethyl)-2-oxoindol-3-ylidene]amino]-2-(4-nitrophenoxy)acetamide Structure](https://image.chemsrc.com/caspic/423/81215-58-9.png)
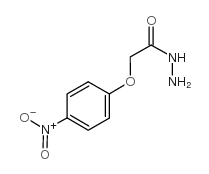
![N-[(E)-[1-[[4-(4-methylphenyl)piperazin-1-yl]methyl]-2-oxoindol-3-ylidene]amino]-2-(4-nitrophenoxy)acetamide Structure](https://image.chemsrc.com/caspic/020/81215-60-3.png)

![N-[[5-methyl-2-oxo-1-(piperidin-1-ylmethyl)indol-3-ylidene]amino]-2-(4-nitrophenoxy)acetamide Structure](https://image.chemsrc.com/caspic/406/81215-63-6.png)
![N-[[5-methyl-1-[[4-(4-methylphenyl)piperazin-1-yl]methyl]-2-oxoindol-3-ylidene]amino]-2-(4-nitrophenoxy)acetamide Structure](https://image.chemsrc.com/caspic/368/81215-64-7.png)