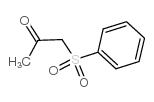
|
~78% |
|
~77% |
|
~% |
|
~% |
|
~22% |
|
~15% |
|
~% |
![E-[[2-(2-propenyloxy)-1-propenyl]sulfonyl]benzene Structure](https://image.chemsrc.com/caspic/321/109787-36-2.png)
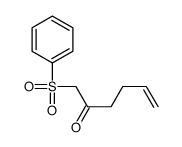
![[[2-(2-propenyloxy)-2-propenyl]sulfonyl]benzene Structure](https://image.chemsrc.com/caspic/359/109787-35-1.png)
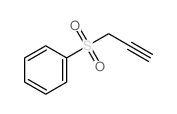
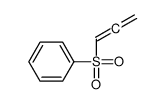
![[[2-(2-butenyloxy)-2-propenyl]sulfonyl]benzene Structure](https://image.chemsrc.com/caspic/245/109787-38-4.png)