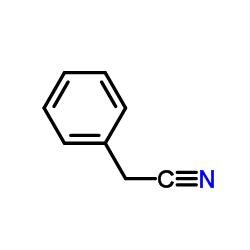
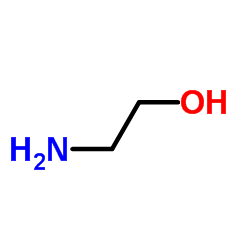
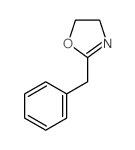
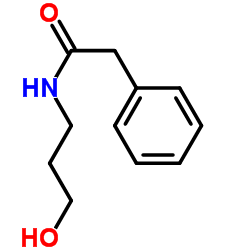
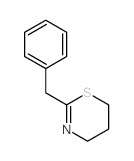
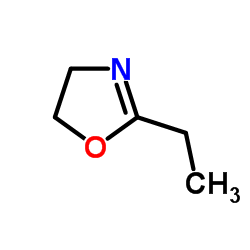
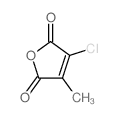
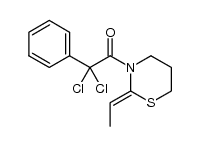
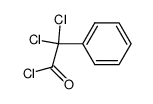
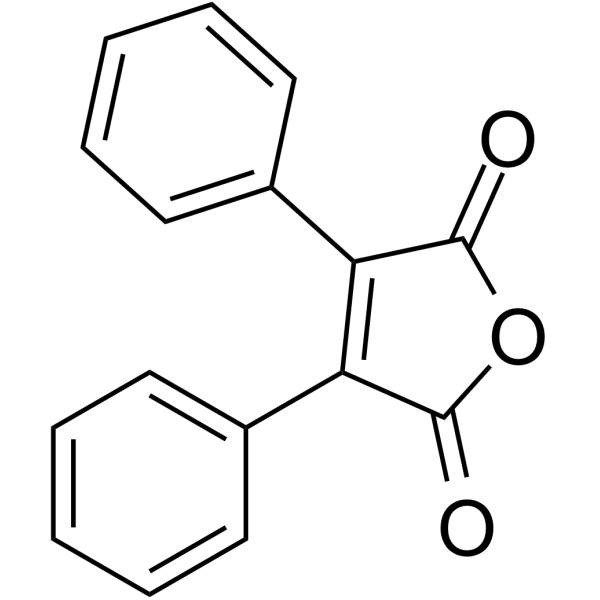
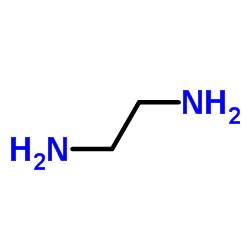
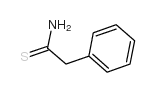
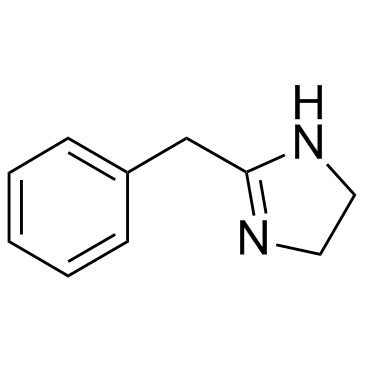
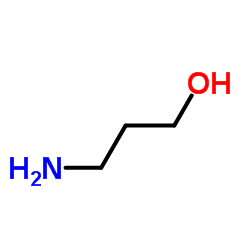
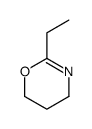
|
~35% |
|
~% |
|
~73% |
|
~19% |
|
~64% |
|
~% |
|
~75% |
|
~99% |
|
~68% |