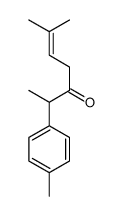
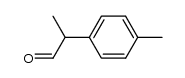
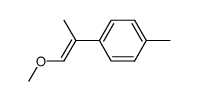
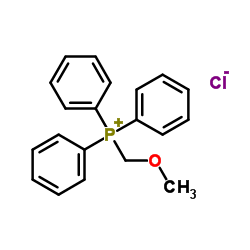
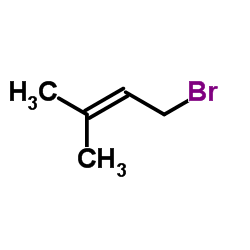
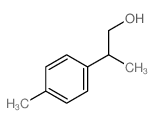
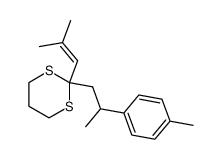
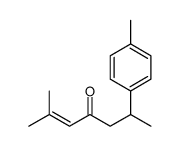
|
~76% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~% |
|
~% |
|
~57% |
|
~% |
|
~% |
|
~% |
![2-(3-methyl-2-butenyl)-2-[1-(4-tolyl)-ethyl]-1,3-dithiane Structure](https://image.chemsrc.com/caspic/111/75316-59-5.png)