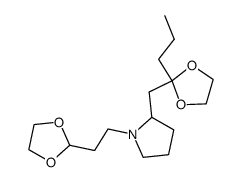
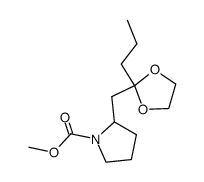
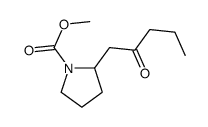
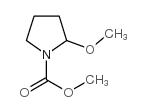
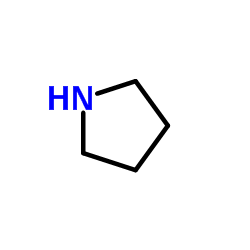
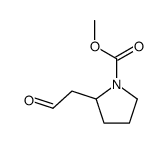
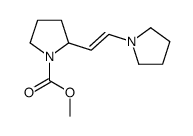
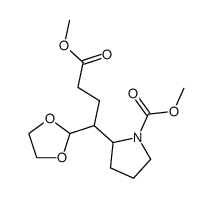
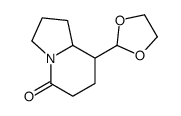
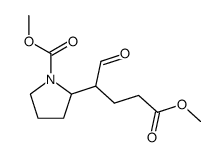
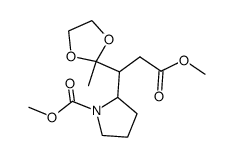
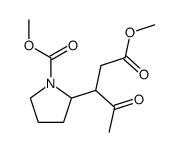
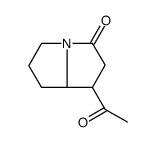
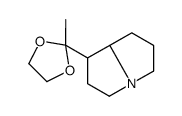
|
~51% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~0% |
|
~% |
|
~% |
|
~% |
|
~4% |
|
~% |
|
~% |
|
~% |
|
~78% |
|
~% |
|
~98% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~69% |
|
~% |
|
~% |
|
~% |
|
~61% |
|
~% |
|
~87% |
|
~% |
|
~% |
|
~95% |
|
~% |
|
~% |
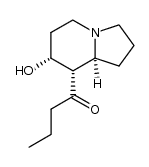


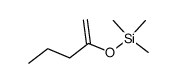
![2-[(2-propyl-1,3-dioxolan-2-yl)methyl]pyrrolidine Structure](https://image.chemsrc.com/caspic/135/88001-30-3.png)