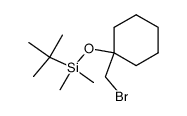
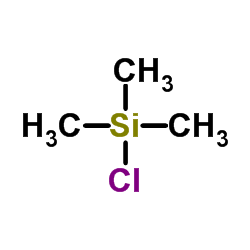
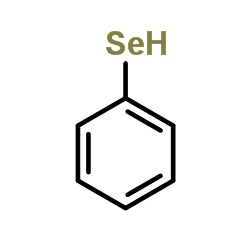
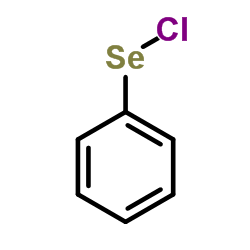
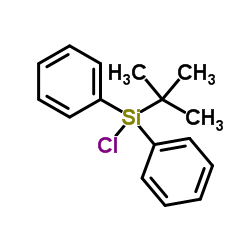
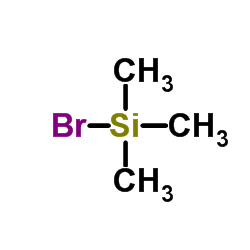|
~78% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~90% |
|
~54% |
|
~% |
|
~67% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~84% |
|
~97% |
|
~99% |
|
~74% |
|
~% |
|
~% |
|
~% |
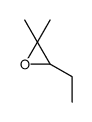

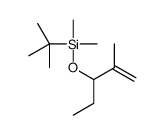
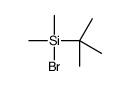


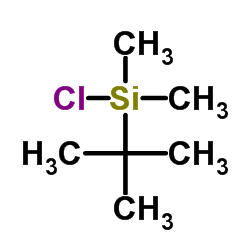
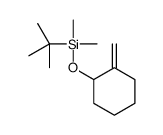
![7-Oxabicyclo[4.1.0]heptane,1-methyl Structure](https://image.chemsrc.com/caspic/499/1713-33-3.png)
![6-[(tert-butyldimethylsilyl)oxy]-1-methylcyclohex-1-ene Structure](https://image.chemsrc.com/caspic/291/72726-55-7.png)

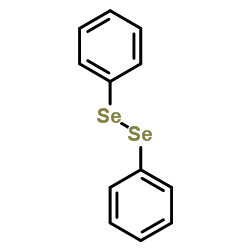
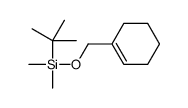

![1-Oxaspiro[2.5]octane Structure](https://image.chemsrc.com/caspic/109/185-70-6.png)