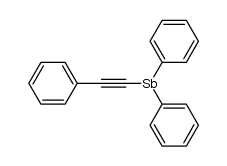
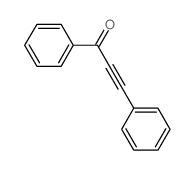
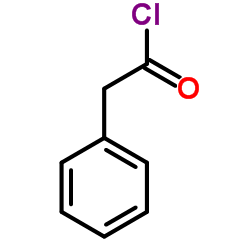
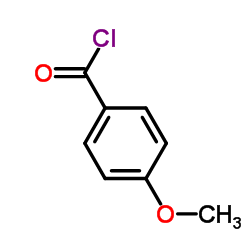
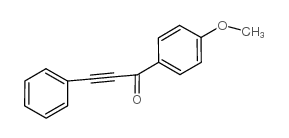
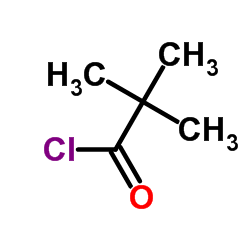
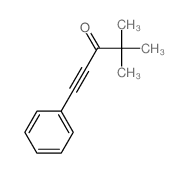
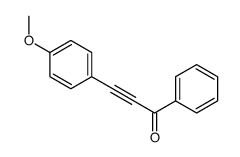
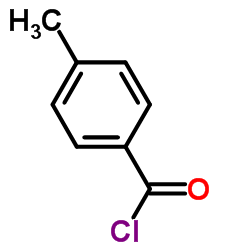
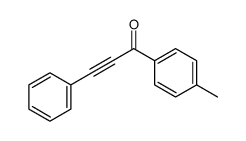
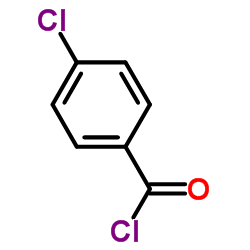
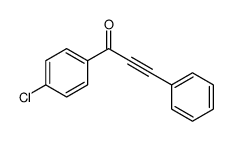
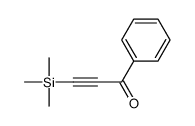
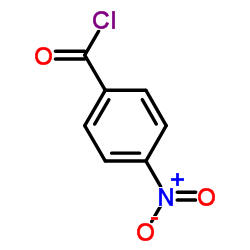
|
~86% |
|
~46% |
|
~75% |
|
~55% |
|
~64% |
|
~74% |
|
~83% |
|
~66% |
|
~74% |