|
~73% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
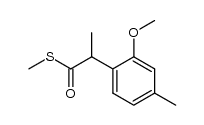
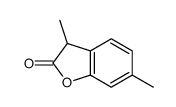
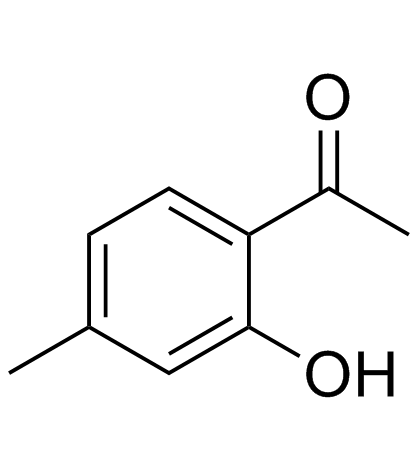
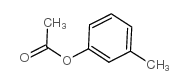
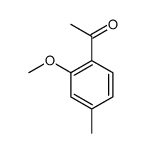

![2-[4-methyl-2-(methoxy)phenyl]-1-tetrahydro-2H-1,4-oxazin-4-yl-1-ethanthione Structure](https://image.chemsrc.com/caspic/230/221354-43-4.png)