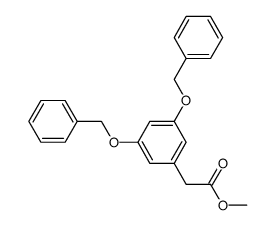
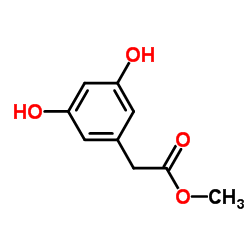
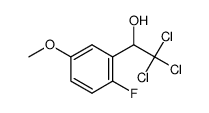
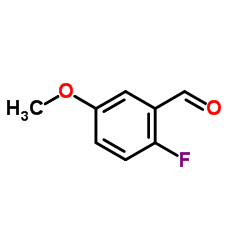
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~84% |
|
~% |
|
~% |
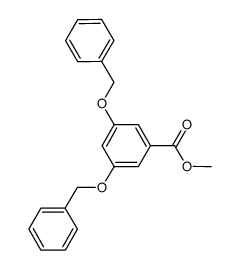
![2-[3,5-bis(phenylmethoxy)phenyl]acetyl chloride Structure](https://image.chemsrc.com/caspic/429/65690-33-7.png)