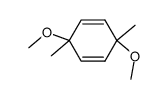
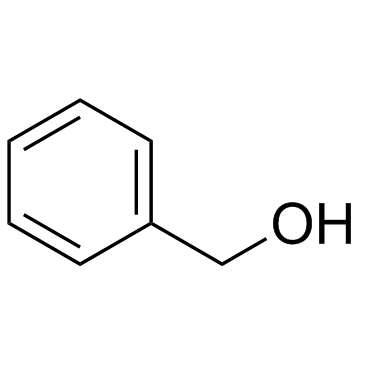
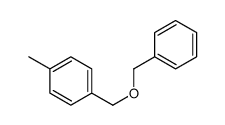
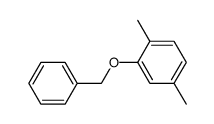
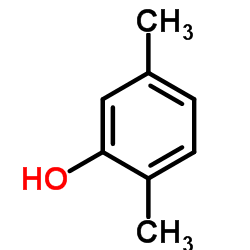
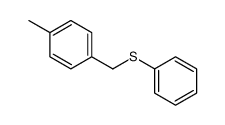
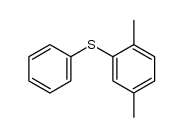
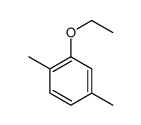
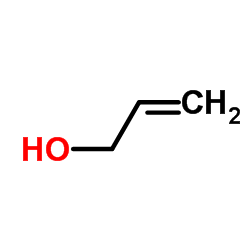
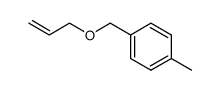
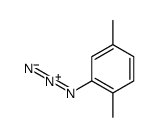
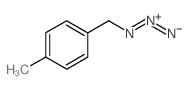
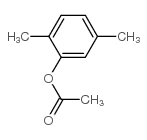
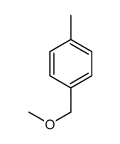
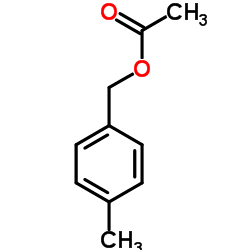
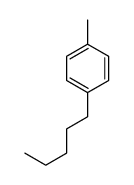
|
~60% |
|
~89% |
|
~16% |
|
~32% |
|
~63% |
|
~40% |
|
~35% |
|
~3%
Detail
|
|
~93% |