|
~37% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~96% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~85% |
|
~99% |
|
~99% |
|
~% |
|
~% |
|
~% |
|
~72% |
|
~% |
![2,5-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-ol Structure](https://image.chemsrc.com/caspic/048/87046-36-4.png)
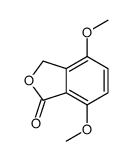
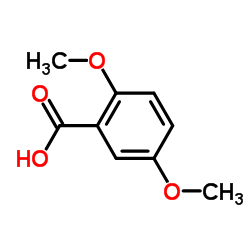
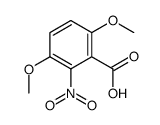
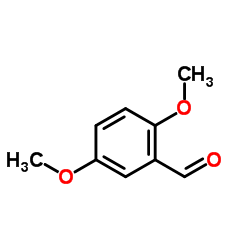

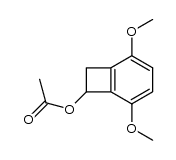
![BICYCLO[4.2.0]OCTA-1,3,5,7-TETRAEN-7-OL Structure](https://image.chemsrc.com/caspic/232/35447-99-5.png)
![7-chlorobicyclo[4.2.0]octa-1,3,5-triene Structure](https://image.chemsrc.com/caspic/466/61599-88-0.png)
![7,7-dichloro-2,5-dimethoxybicyclo[4.2.0]octa-1,3,5-triene Structure](https://image.chemsrc.com/caspic/208/75833-44-2.png)
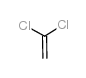

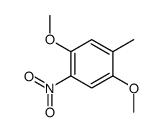
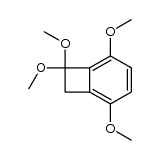
![2,5-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-one Structure](https://image.chemsrc.com/caspic/170/75833-45-3.png)
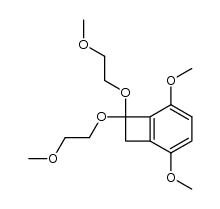
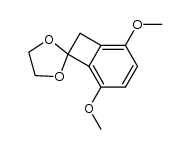
![Bicyclo[4.2.0]octa-1,3,5-trien-7-one Structure](https://image.chemsrc.com/caspic/187/3469-06-5.png)