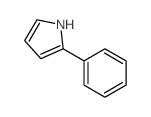
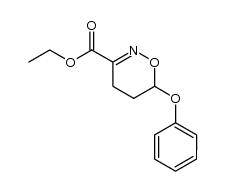
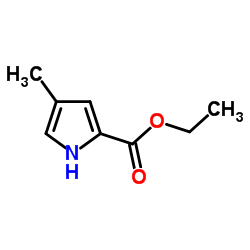
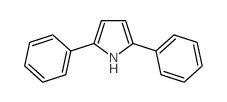
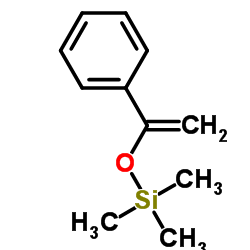
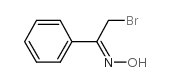
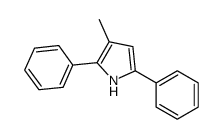
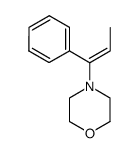
|
~89% |
|
~95% |
|
~55% |
|
~58% |
|
~18% |
|
~% |
|
~% |
|
~68% |
|
~% |
|
~% |
|
~% |
|
~% |