|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

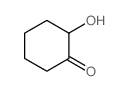
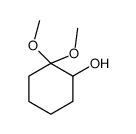

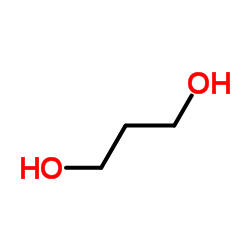
![11-chloro-1,5-dioxaspiro[5.5]undecane Structure](https://image.chemsrc.com/caspic/383/67592-52-3.png)
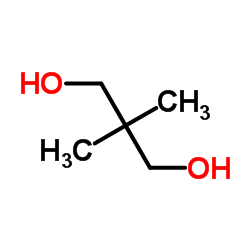
![11-chloro-3,3-dimethyl-1,5-dioxaspiro[5.5]undecane Structure](https://image.chemsrc.com/caspic/408/73777-20-5.png)

![11-bromo-3,3-dimethyl-1,5-dioxaspiro[5.5]undecane Structure](https://image.chemsrc.com/caspic/332/73777-22-7.png)
![5-chloro-7,12-dioxaspiro[5.6]dodecane Structure](https://image.chemsrc.com/caspic/228/84657-99-8.png)
![5-bromo-7,12-dioxaspiro[5.6]dodecane Structure](https://image.chemsrc.com/caspic/107/84658-00-4.png)
![5-methoxy-7,12-dioxaspiro[5.6]dodecane Structure](https://image.chemsrc.com/caspic/031/84658-02-6.png)
![7,12-dioxaspiro[5.6]dodecan-1-ol Structure](https://image.chemsrc.com/caspic/069/84658-01-5.png)
