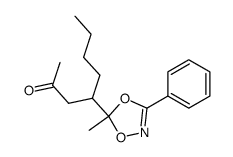
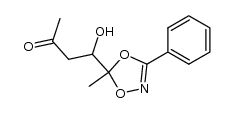
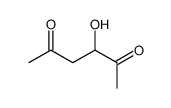
|
~% |
|
~0% |
|
~% |
|
~87% |
|
~% |
![4c-(5-methyl-3-phenyl-[1,4,2]dioxazol-5-yl)-but-3-en-2-one Structure](https://image.chemsrc.com/caspic/124/66310-18-7.png)