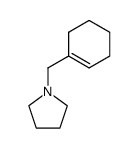
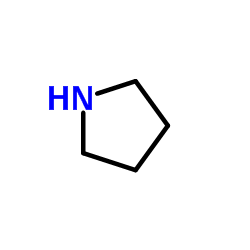
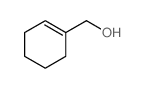
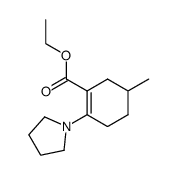
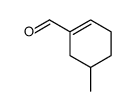
|
~52% |
|
~% |
|
~14% |
|
~17% |
|
~56% |
|
~% |
|
~8% |
|
~% |
|
~9% |
|
~51% |

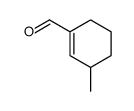
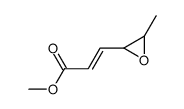
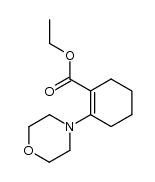

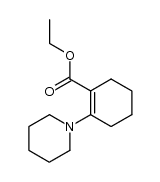
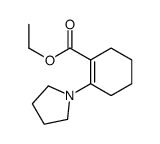
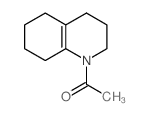
![1-(2-azabicyclo[4.3.0]non-10-en-2-yl)ethanone Structure](https://image.chemsrc.com/caspic/461/71866-22-3.png)