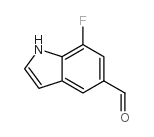
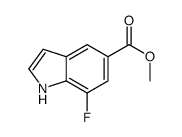
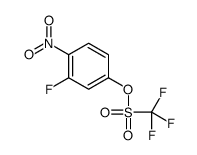
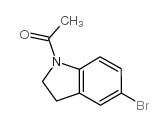
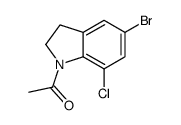
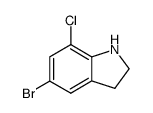
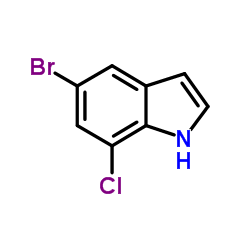
|
~80% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~86% |
|
~% |
|
~% |
|
~53% |
|
~97% |