|
~76% |
|
~91% |
|
~35% |
|
~% |
|
~83% |
|
~83% |
|
~98% |
![5-[(4-bromophenyl)methyl]-2,2-dimethyl-1,3-dioxane-4,6-dione Structure](https://image.chemsrc.com/caspic/465/198494-75-6.png)
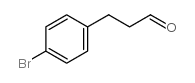
![5-(4-nitrobenzyl)-2,2-dimethyl-[1,3]dioxane-4,6-dione Structure](https://image.chemsrc.com/caspic/434/154317-89-2.png)
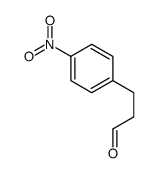
![2,2-dimethyl-5-[(4-nitrophenyl)methylidene]-1,3-dioxane-4,6-dione Structure](https://image.chemsrc.com/caspic/160/15795-62-7.png)
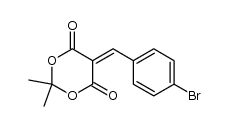
![1,3-Dioxane-4,6-dione, 5-[(3,4-dimethoxyphenyl)methylene]-2,2-dimethyl- (en) Structure](https://image.chemsrc.com/caspic/352/67101-91-1.png)
![5-[(3,4-dimethoxyphenyl)methyl]-2,2-dimethyl-1,3-dioxane-4,6-dione Structure](https://image.chemsrc.com/caspic/189/154317-78-9.png)
![5-[(4-methoxyphenyl)methyl]-2,2-dimethyl-1,3-dioxane-4,6-dione Structure](https://image.chemsrc.com/caspic/346/61958-46-1.png)
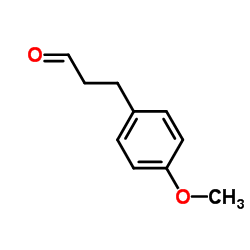
![5-[(4-METHOXYPHENYL)METHYLENE]-2,2-DIMETHYL-1,3-DIOXANE-4,6-DIONE Structure](https://image.chemsrc.com/caspic/291/15795-54-7.png)